Scinderin, an Actin Severing and Nucleating Protein: Molecular Structure and Possible Roles in Cell Secretion, Maturation, Differentiation and Cancer
REVIEW
Abstract
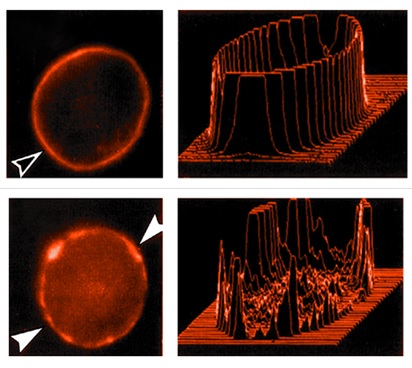
Scinderin is a filamentous actin-severing and capping protein. Segmental deletion and point-mutation studies have shown that this scinderin actin-severing activity resides in its NH2 terminal half, precisely in domains 1 and 2. The actin nucleating activity of scinderin is in its domain 5. Scinderin’s F-actin severing activity plays a role in cell secretion. In secretory cells, scinderin is highly concentrated in the cell cortex, showing a distribution similar to that of filamentous actin. Two PIP2-binding sites have been described in scinderin domains 1 and 2. PIP2 inhibits the actin-severing activity of the protein. Several approaches [recombinant scinderin, antisence oligonucleotide, gene promoter activation, vector-mediated expression of scinderin active domains] were used to prove the role of scinderin in secretion. The promoter of SCIN, the scinderin gene, has been characterized and its responsive elements have been described. The activation of the SCIN promoter by two ligands of the aryl hydrocarbon receptor [AhR] transcription factor has been shown. This activation increased scinderin expression with a resulting increase in stimulation-induced cortical actin disassembly and exocytosis. A role for scinderin in the life cycle of megakaryocytes has also been demonstrated. Scinderin seems to be involved in the maturation, differentiation and apoptosis processes of these cells, releasing newly formed platelets. This completes the life cycle of a megakaryocyte. A role for scinderin in cancer is also emerging. The SCIN gene is silenced in megakaryocyte leukemia cells and its re-expression induces cell maturation, differentiation, apoptosis, as well as the release of platelet-like particles and anti-tumor effects. These are always accompanied by changes in cell morphology due to cytoskeleton re-arrangements. Scinderin over-expression is necessary to see anti–proliferation effects, which are always accompanied by maturation and differentiation. If there were methods to differentiate other types of tumor cells, a new area of treatment would be developed by inducing “cell maturation and/or differentiation”.